How to find oxidation number To determine the oxidation quantity of an atom within a compound or ion, several tips can be accompanied. Firstly, for factors in their elemental form, the oxidation wide variety is 0. In easy ions, the oxidation quantity equals the fee of the ion. Certain factors have fixed oxidation numbers in compounds; for instance, oxygen normally has an oxidation wide variety of -2, hydrogen is normally 1, and fluorine is always -1. The sum of oxidation numbers in a impartial compound is zero, and in a polyatomic ion, it equals the fee of the ion. By thinking about these rules and making use of algebraic manipulation when essential, possible deduce the oxidation wide variety of the atom of interest based on recognised oxidation numbers of different atoms and the overall price of the compound or ion. This technique is crucial in understanding redox reactions, where oxidation numbers alternate, and in balancing chemical equations correctly.
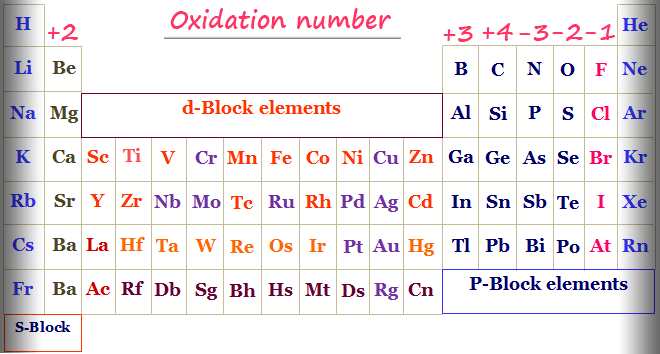
Rules for Assigning Oxidation Numbers
Here are the regulations for assigning oxidation numbers:
- Elemental Form: In their elemental shape, atoms have an oxidation wide variety of 0. For example, O2, H2, and Na have oxidation numbers of 0.
- Simple Ions: The oxidation range of an atom in a simple ion is identical to the price of the ion. For instance, inside the sodium ion (Na ), the oxidation variety of sodium is 1, at the same time as within the chloride ion (Cl-), the oxidation quantity of chlorine is -1.
- Standard Oxidation Numbers: Some elements have general oxidation numbers in maximum compounds. For example, oxygen typically has an oxidation quantity of -2, hydrogen commonly has 1, and fluorine always has -1.
- Neutral Compounds: In impartial compounds, the sum of the oxidation numbers of all atoms equals 0. For example, in water (H2O), the oxidation wide variety of hydrogen is 1, and for oxygen, it’s far -2, ensuring a complete of 0.
- Polyatomic Ions: In polyatomic ions, the sum of the oxidation numbers of all atoms equals the price of the ion. For example, in the sulfate ion (SO4^2-), the sum of the oxidation numbers of sulfur and oxygen atoms equals -2.
- Algebraic Manipulation: Use algebraic manipulation to decide the oxidation range of an detail if necessary, considering the recognized oxidation numbers of different atoms and the overall rate of the compound or ion.
How to find oxidation number
To find the oxidation number of an atom in a compound or ion, follow these steps:
- Identify the Compound: Determine the compound or ion for which you want to find the oxidation number of a specific atom.
- Know the Rules: Understand the rules for assigning oxidation numbers, which include guidelines based on the element’s behavior and its role in the compound.
- Elemental Form: Elements in their elemental form have an oxidation number of zero. For example, oxygen (O2) and hydrogen (H2) both have oxidation numbers of zero.
- Simple Ions: In simple ions, the oxidation number is equal to the charge of the ion. For example, the oxidation number of sodium (Na) in the sodium ion (Na+) is +1, while the oxidation number of chlorine (Cl) in the chloride ion (Cl-) is -1.
- Standard Oxidation Numbers: Some elements have standard oxidation numbers in most compounds. For instance, oxygen typically has an oxidation number of -2, hydrogen usually has +1, and fluorine always has -1.
- Neutral Compounds: In neutral compounds, the sum of the oxidation numbers of all atoms equals zero. For example, in water (H2O), the oxidation number of hydrogen is +1, and for oxygen, it is -2, ensuring a total of zero.
- Polyatomic Ions: In polyatomic ions, the sum of the oxidation numbers of all atoms equals the charge of the ion.
- Algebraic Manipulation: Use algebraic manipulation to determine the oxidation number of an element if necessary, considering the known oxidation numbers of other atoms and the overall charge of the compound or ion.
Role of oxidation numbers in chemical processes
Oxidation numbers play a crucial role in various chemical processes, providing valuable information about the distribution of electrons within compounds and ions. Here’s how oxidation numbers contribute to understanding and predicting chemical reactions:
- Redox Reactions: Oxidation numbers help identify redox (oxidation-reduction) reactions, where electrons are transferred between atoms. In these reactions, one substance loses electrons (oxidation) while another gains electrons (reduction). By tracking changes in oxidation numbers, we can determine which atoms are oxidized and which are reduced.
- Balancing Equations: Oxidation numbers are essential for balancing chemical equations, especially in redox reactions. Balancing involves ensuring that the total charge and total mass remain unchanged before and after the reaction. By adjusting coefficients and oxidation numbers, we can balance equations accurately.
- Identifying Oxidizing and Reducing Agents: Oxidation numbers help identify oxidizing and reducing agents in redox reactions. The oxidizing agent causes another substance to lose electrons (undergo oxidation) by itself being reduced, while the reducing agent causes another substance to gain electrons (undergo reduction) by itself being oxidized.
- Stoichiometry: Oxidation numbers assist in stoichiometric calculations, such as determining the quantities of reactants and products in a chemical reaction. They provide insights into the electron transfer processes occurring during reactions, aiding in the calculation of reaction yields and product formation.
- Predicting Reactivity: Knowledge of oxidation numbers helps predict the reactivity of substances in chemical reactions. Elements with higher oxidation numbers tend to be more reactive because they are more likely to undergo reduction (gain electrons) to attain a stable configuration.
- Understanding Bonding: Oxidation numbers reflect the sharing or transfer of electrons in chemical bonds. In covalent compounds, oxidation numbers indicate the distribution of shared electrons, while in ionic compounds, they reveal the transfer of electrons between ions.
Tips for students to understand and calculate oxidation numbers
Here are some tips to help students understand and calculate oxidation numbers effectively:
- Learn the Rules: Familiarize yourself with the rules for assigning oxidation numbers, including guidelines for elements in their elemental form, in simple ions, and in compounds.
- Practice Regularly: Practice assigning oxidation numbers to atoms in various compounds and ions regularly. The more you practice, the more confident you’ll become in identifying oxidation numbers accurately.
- Memorize Common Oxidation Numbers: Memorize the common oxidation numbers for elements such as oxygen (-2), hydrogen (+1), and fluorine (-1). Knowing these common oxidation numbers will speed up the process of assigning oxidation numbers in compounds.
- Work Step-by-Step: Break down the process of calculating oxidation numbers into smaller steps. Start by identifying the type of compound or ion, then apply the relevant rules systematically to assign oxidation numbers to each atom.
- Check Your Work: Double-check your calculations to ensure accuracy. Verify that the sum of oxidation numbers in a neutral compound is zero and that it equals the charge of a polyatomic ion.
- Use Examples: Work through examples provided in textbooks, online resources, or class notes. Seeing worked examples can help reinforce understanding and clarify any confusion about assigning oxidation numbers.
- Seek Help When Needed: Don’t hesitate to ask your teacher, classmates, or online resources for help if you’re struggling to understand or calculate oxidation numbers. Sometimes discussing problems with others can lead to a better understanding.
- Apply to Real-World Examples: Connect the concept of oxidation numbers to real-world examples and applications in chemistry. Understanding how oxidation numbers relate to redox reactions, balancing equations, and predicting reactivity can make the concept more tangible and relevant.
- Create Mnemonics: Use mnemonic devices or memory aids to remember key concepts or rules related to oxidation numbers. Mnemonics can help you recall information more easily during exams or when solving problems.
- Stay Persistent: Don’t get discouraged if you find oxidation numbers challenging at first. Keep practicing, asking questions, and seeking clarification, and with time and effort, you’ll improve your understanding and proficiency in calculating oxidation numbers.
Conclusion
In end, understanding and How to find oxidation number calculating oxidation numbers is a essential skill in chemistry that plays a important function in diverse elements of chemical evaluation, inclusive of redox reactions, stoichiometry, and reaction prediction. By following the policies for assigning oxidation numbers, practicing frequently, and applying trouble-fixing strategies, students can broaden skillability in this area. Regular exercise, coupled with a strong expertise of the underlying principles, allows college students to as it should be assign oxidation numbers and navigate complicated chemical approaches with confidence. With determination, staying power, and the utilization of powerful look at techniques, college students can master the idea of oxidation numbers and beautify their overall comprehension of chemistry.a
FAQs
Q: 1 What is an oxidation number?
Ans:: An oxidation number is a theoretical concept representing the apparent charge of an atom in a compound or ion, based on a set of rules. It indicates the number of electrons gained or lost by an atom to form the compound or ion.
Q: 2How do I determine the oxidation number of an element?
Ans: To determine the oxidation number, follow specific rules based on the element’s behavior and its role in the compound or ion. For example, elements in their elemental form have an oxidation number of zero, while simple ions have oxidation numbers equal to their charges.
Q:3 What are some common oxidation numbers for elements?
Ans: Some elements have standard oxidation numbers in most compounds. For instance, oxygen typically has an oxidation number of -2, hydrogen usually has +1, and fluorine always has -1. However, oxidation numbers can vary depending on the compound or ion.
Q: 4 Why are oxidation numbers important in chemistry?
Ans: Oxidation numbers are crucial for understanding redox reactions, where electrons are transferred between atoms. They help in balancing chemical equations, identifying oxidizing and reducing agents, and predicting the reactivity of substances in chemical reactions.






