Chemistry Sample Paper Class 11 : With the help of our website, which has an extensive collection of Previous Paper of Class 11 CBSE Board, you may successfully prepare for your Class 11 CBSE Board Exam. Get access to a large selection of past exam questions that have been carefully chosen to cover subjects related to the Class 11 CBSE Board course . Download practice tests in several formats, such as multiple-choice questions (MCQs) and descriptive questions, to help you prepare for exams and increase your confidence. With the help of our platform, you can revise with concentrate and recognize important themes and question formulation trends. Get ongoing help and direction to help you prepare for and pass the CBSE Board Exam.
- Introduction : Chemistry Sample Paper Class 11
- Download : Chemistry Sample Paper Class 11 with Solution
- Syllabus :Chemistry Sample Paper Class 11
- Exam Pattern : Chemistry Sample Paper Class 11
- Significance of Chemistry Sample Paper Class 11
- Tips for Good Preparation : Chemistry Sample Paper Class 11
- FAQs : Chemistry Sample Paper Class 11
Introduction : Chemistry Sample Paper Class 11
An introduction to the Class 11 Chemistry exam. Prepare for a journey through chemistry’s marvels. This exam tests your understanding of molecules, reactions, and the fundamental principles of matter.
Purpose
This exam assesses your understanding of basic concepts in chemistry, including molecules, processes, and the laws of matter. It seeks to evaluate how well you comprehend and apply these ideas. I wish you luck as you present your expertise and critical thinking!
Components
Test structure
The exam is divided into sections that cover a variety of topics related to chemistry, such as molecules, processes, and the basic concepts of matter. There are questions in every section meant to test your comprehension and application of these ideas. A range of formats, including multiple choice, short answer, and problem-solving, should be anticipated. Make the most of your time so that you can properly handle each section. This methodical methodology guarantees a thorough evaluation of your abilities and knowledge in a variety of chemistry-related areas. I wish you luck as you navigate through the various components of the test!
Exam Preparation
Review important ideas such as molecules, processes, and the basic principles of matter to help you prepare for the test. Practice answering questions and finding solutions to issues that resemble those you’ll face. Make use of study tools such as online resources, notes, and textbooks. Put comprehension ahead of memory in order to apply knowledge successfully. Make good use of your time so that you can cover everything. Remain composed and self-assured since you’ve done your homework. I wish you well as you study for your test!
Conclusion
To sum up, this test is designed to provide a thorough assessment of your comprehension of key chemical ideas such as molecules, reactions, and the building blocks of matter. You prepare yourself to face the obstacles by doing thorough preparation and problem-solving exercises. Always remember to take a deliberate approach to every issue and use your time wisely. Have faith in your readiness and seize the chance to show off your chemical skills. Wishing you luck on the test and success with your endeavors!
Download : Chemistry Sample Paper Class 11 with Solution
| Title | Question Paper | Solution |
|---|---|---|
| Chemistry Sample Paper Class 11 | Download Here | Download Here |
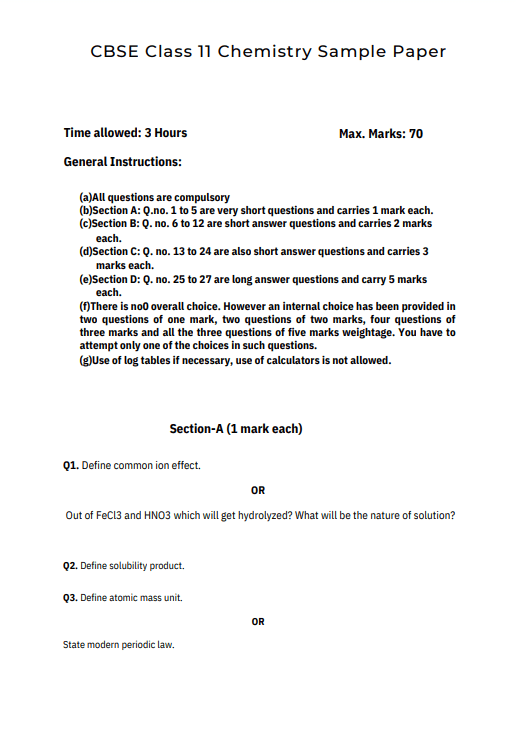


Syllabus : Chemistry Sample Paper Class 11
|
S. No. |
Unit |
No. of Periods |
Marks |
|
1. |
Some Basic Concepts of Chemistry |
12 |
7 |
|
2. |
Structure of Atom |
14 |
9 |
| 3. |
Classification of Elements and Periodicity in Properties |
8 |
6 |
| 4. |
Chemical Bonding and Molecular Structure |
14 |
7 |
| 5. |
Chemical Thermodynamics |
16 |
9 |
| 6. |
Equilibrium |
14 |
7 |
|
7. |
Redox Reactions |
6 |
4 |
| 8. |
Organic Chemistry: Some basic Principles and Techniques |
14 |
11 |
|
9. |
Hydrocarbons |
12 |
10 |
|
Total |
70 |
||
Unit-wise CBSE Class 11 Chemistry Syllabus
The CBSE Class 11 Chemistry Syllabus 2024–25 has been designed so that pupils would grasp the principles and not face any challenges in Class 12. The official PDF and the CBSE 11th Chemistry Syllabus 2024–25 may be found here. Before exams, students should review every chapter in the CBSE Class 11 Chemistry Syllabus 2024–25 and make an effort to cover it completely.
Unit |
Chapter |
Topics |
|---|---|---|
| I. |
Some Basic Concepts of Chemistry |
General Introduction: Importance and scope of Chemistry. Nature of matter, laws of chemical combination, Dalton’s atomic theory: the concept of elements, atoms, and molecules. Atomic and molecular masses, mole concept and molar mass, percentage composition, empirical and molecular formula, chemical reactions, stoichiometry, and calculations based on stoichiometry. |
| II. |
Structure of Atom |
Discovery of Electron, Proton and Neutron, atomic number, isotopes and isobars. Thomson’s model and its limitations. Rutherford’s model and its limitations, Bohr’s model and its limitations, concept of shells and subshells, dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, concept of orbitals, quantum numbers, shapes of s, p and d orbitals, rules for filling electrons in orbitals – Aufbau principle, Pauli’s exclusion principle and Hund’s rule, electronic configuration of atoms, stability of half-filled and filled orbitals. |
| III. |
Classification of Elements and Periodicity |
Significance of classification, a brief history of the development of the periodic table, modern periodic law and the present form of the periodic table, periodic trends in properties of elements -atomic radii, ionic radii, inert gas radii, Ionization enthalpy, electron gain enthalpy, electronegativity, valency. Nomenclature of elements with atomic number greater than 100. |
| IV. |
Chemical Bonding and Molecular Structure |
Valence electrons, ionic bond, covalent bond, bond parameters, Lewis’s structure, polar character of covalent bond, covalent character of ionic bond, valence bond theory, resonance, geometry of covalent molecules, VSEPR theory, concept of hybridization, involving s, p and d orbitals and shapes of some simple molecules, molecular orbital theory of homonuclear diatomic molecules (qualitative idea only), Hydrogen bond. |
| V. |
Chemical Thermodynamics |
Concepts of Systems and types of systems, surroundings, work, heat, energy, extensive and intensive properties, and state functions. The first law of thermodynamics -internal energy and enthalpy, heat capacity and specific heat, measurement of ΔU and ΔH, Hess’s law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution and dilution. The second law of Thermodynamics (brief introduction) Introduction of entropy as a state function, Gibb’s energy change for spontaneous and non-spontaneous processes, and criteria for equilibrium. Third law of thermodynamics (brief introduction). |
| VI. |
Equilibrium |
Equilibrium in physical and chemical processes, dynamic nature of equilibrium, the law of mass action, equilibrium constant, factors affecting equilibrium – Le Chatelier’s principle, ionic equilibrium- ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of polybasic acids, acid strength, the concept of pH, hydrolysis of salts (elementary idea), buffer solution, Henderson Equation, solubility product, common ion effect (with illustrative examples). |
| VII. |
Redox Reactions |
Concept of oxidation and reduction, redox reactions, oxidation number, balancing redox reactions, in terms of loss and gain of electrons and change in oxidation number, applications of redox reactions. |
| VIII. |
Organic Chemistry – Some Basic Principles and Techniques |
General introduction, methods of purification, qualitative and quantitative analysis, classification and IUPAC nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and hyperconjugation. Homolytic and heterolytic fission of a covalent bond: free radicals, carbocations, carbanions, electrophiles and nucleophiles, types of organic reactions. |
| IX. |
Hydrocarbons: |
Classification of Hydrocarbons Aliphatic Hydrocarbons: Alkanes – Nomenclature, isomerism, conformation (ethane only), physical properties, chemical reactions including free radical mechanism of halogenation, combustion and pyrolysis. Alkenes – Nomenclature, the structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation, chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov’s addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition. Alkynes – Nomenclature, the structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of – hydrogen, halogens, hydrogen halides, and water. Aromatic Hydrocarbons: Introduction, IUPAC nomenclature, benzene: resonance, aromaticity, chemical properties: mechanism of electrophilic substitution. Nitration, sulphonation, halogenation, Friedel Craft’s alkylation and acylation, directive influence of the functional group in monosubstituted benzene. Carcinogenicity and toxicity |
Exam Pattern : Chemistry Sample Paper Class 11
| Exam Component | Description |
|---|---|
| Objective Type Questions | – Multiple Choice Questions (MCQs) – True/False statements – Match the following type questions |
| Short Answer Questions | – Definition-based questions – Brief explanations of laws, principles, and theories – Description of limitations of atomic models – Differentiation of chemical bonds |
| Numerical Problems | – Stoichiometry problems – Calculation of empirical and molecular formulas – Determination of atomic and molar masses – Energy change calculations |
| Long Answer Questions | – Explanation of atomic models and their limitations – Discussion on periodic table and trends in elements – Elaboration on chemical equilibrium principles |
| Practical/Experimental | – Interpretation and prediction of experimental outcomes – Analysis of chemical reactions based on observations |
| Diagram/Structure-Based | – Labeling and identification of atomic structures – Drawing Lewis structures and molecular geometries – Illustrating organic reaction mechanisms |
| Essay Questions (optional) | – Critical analysis of chemical concepts or theories – Comparative analysis of atomic models – Discussion on environmental impacts of chemical reactions |
Practical for Class 11 Chemistry
| Practical Component | Marks |
|---|---|
| Volumetric Analysis | 8 |
| Salt Analysis | 8 |
| Content Based Experiment | 6 |
| Project Work | 4 |
| Class Record and Viva | 4 |
| Total | 30 |
Significance of Chemistry Sample Paper Class 11
The significance of Chemistry Sample Paper Class 11 lies in their ability to serve as valuable study resources for candidates preparing for the CBSE Board examination. Here are some key reasons why these question papers are important:
Exam Blueprint Revealed:
The actual exam is modeled by these papers. You can learn a lot about the arrangement of the questions, the relative importance of the various areas on the syllabus, and even the degree of difficulty by carefully examining them. This enables you to customize your study and give priority to the subjects that need greater attention.
Improving Your Skills:
Using past year papers for practice is similar to taking practice exams in a real exam setting. You get to put your speed, accuracy, and conceptual understanding to the test in a virtual setting. This assists in determining your areas of strength and weakness prior to the exam, enabling you to improve your strategy and reinforce your comprehension of important subjects.
Building Exam Stamina:
The Class 11 CBSE Board exam may have a time limit, therefore success depends on your ability to manage your time well. You can improve your endurance and time management abilities for the test by using previous year’s papers. You can learn to pace yourself, prioritize questions, and stay away from becoming bogged down on any one problem by practicing in a timed environment.
Increasing Confidence:
Completing last year’s papers successfully boosts your self-assurance and eases exam anxiety. Observing that you can appropriately respond to questions validates your understanding and inspires you to keep trying. Your overall exam performance is significantly impacted by this positive reinforcement.
Finding Recurring Patterns:
Although the precise questions won’t be asked again, reviewing previous exams frequently identifies patterns in the subjects and question types that are asked again. This enables you to create focused strategies for answering the kinds of questions you might encounter on the actual exam by anticipating their types.
It’s like having a secret weapon when you use the Chemistry Sample Paper Class 11 in your preparing approach. They sharpen your abilities, give you confidence boosts, and offer priceless insights, all of which considerably raise your chances of succeeding on test day.
Tips for Good Preparation : Chemistry Sample Paper Class 11
Recognize the test and syllabus:
Visit the CBSE Board website to download the official announcement and curriculum.
Recognize the format of the exam (number of sections, weighted scores, time allotment).
Learn everything there is to know about the subjects included on the curriculum for each area.
Create a Timetable and Study Plan:
Make a realistic study schedule with time allotted for each section based on the syllabus and your preferred method of learning.
Establish study times on a daily or weekly basis, and try your best to maintain them.
Be adaptable and make necessary changes to your plan, but consistency is essential.
Establish a Robust Base:
Pay close attention to the fundamental ideas in each area, paying particular attention.
Learn the fundamental, shortcuts, and approaches to solving problems.
Make Use of Educational Resources
Make use of top-notch study resources, such as online courses, textbooks, and coaching materials (if necessary).
Exam patterns and time management exercises can be learned by looking at previous year’s question papers and practice exams.
Consistent Practice:
Every day, complete practice questions from different sources.
Prioritize precision while progressively picking up speed.
Examine your errors and determine what needs to be improved.
By following these tips and dedicating yourself to consistent preparation, you can significantly increase your chances of success in the Class 11 (Chemistry Sample Paper Class 11) CBSE Board Exam. Remember, the key is to start early, work hard, and stay focused on your goal.
FAQs : Chemistry Sample Paper Class 11
Q1: What are the fundamental concepts of chemistry?
A1: The fundamental concepts of chemistry include matter and its nature, the structure of the atom, the periodic table, chemical bonding, states of matter, thermodynamics, and chemical equilibrium.
Q2: What is the significance of the periodic table in chemistry?
A2: The periodic table organizes elements based on their atomic number, electron configuration, and recurring chemical properties. It helps in predicting the properties of elements and understanding their relationships.
Q3: Explain the different types of chemical bonding.
A3: Chemical bonding includes ionic, covalent, and metallic bonding. Ionic bonding involves the transfer of electrons, covalent bonding involves the sharing of electrons, and metallic bonding involves a sea of delocalized electrons in a lattice of positive ions.
Q4: What are the different states of matter, and how do they differ?
A4: The three primary states of matter are solid, liquid, and gas. Solids have a fixed shape and volume, liquids have a fixed volume but take the shape of their container, and gases have neither a fixed shape nor volume.
Q5: Explain the concept of stoichiometry.
A5: Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. It involves calculations based on the balanced chemical equation, such as mole ratios, mass-to-mole conversions, and stoichiometric calculations.






