How to balance chemical equations Balancing chemical equations involves ensuring that the number of atoms of each element on the reactant side equals the number on the product side. Start by writing down the unbalanced equation with the correct chemical formulas. Then, adjust coefficients (numbers in front of each compound) to balance the equation. Begin with elements that appear only once on each side and balance them using coefficients. Next, balance elements that appear in multiple compounds by adjusting coefficients until the number of atoms of each element is the same on both sides of the equation. Verify the balance by counting the atoms of each element after adjusting coefficients, ensuring consistency throughout the equation
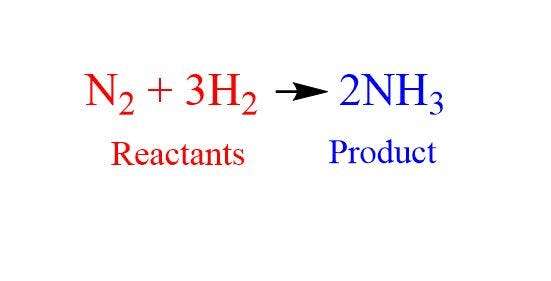
Understanding Chemical Equations
Understanding chemical equations involves several key points:
- Representation: Chemical equations depict chemical reactions using chemical formulas of reactants (substances before the reaction) and products (substances after the reaction).
- Balancing: Equations must be balanced to adhere to the law of conservation of mass, ensuring the same number of atoms of each element on both sides.
- Coefficients: Numbers placed in front of compounds adjust the quantities to balance the equation. These coefficients represent the ratio of molecules or moles involved in the reaction.
- Types of Reactions: Equations can represent different types of reactions, such as synthesis (combination), decomposition, single displacement, double displacement, and combustion.
- Stoichiometry: Involves calculating quantities of reactants and products based on balanced equations and molar ratios, crucial for understanding reaction yields in practical applications.
- Symbols and Notations: Symbols like (s), (l), (g), and (aq) indicate physical states (solid, liquid, gas, aqueous) of substances in reactions, providing additional information about reaction conditions.
Balancing Equations with Simple Examples
Balancing chemical equations ensures that the number of atoms of each element is the same on both sides of the equation. Here are some simple examples and steps to balance them:
Example 1: Combustion of Methane (CH₄) with Oxygen (O₂) to form Carbon Dioxide (CO₂) and Water (H₂O)
Unbalanced Equation: CH4+O2→CO2+H2OCH4+O2→CO2+H2O
Steps to Balance:
- Start with carbon (C) and balance it first. There’s 1 carbon on the left and 1 on the right. CH4+O2→CO2+H2OCH4+O2→CO2+H2O
- Balance hydrogen (H) next. There are 4 hydrogens on the left and 2 on the right. To balance hydrogen, place a coefficient of 2 in front of H₂O. CH4+O2→CO2+2H2OCH4+O2→CO2+2H2O
- Finally, balance oxygen (O). There are 2 oxygens from CO₂ and 2 × 1 = 2 oxygens from H₂O, totaling 4 oxygens on the right. To balance oxygen, place a coefficient of 2 in front of O₂. CH4+2O2→CO2+2H2OCH4+2O2→CO2+2H2O
Now, the equation is balanced with 1 carbon, 4 hydrogens, and 4 oxygens on both sides.
Example 2: Formation of Water (H₂O) from Hydrogen (H₂) and Oxygen (O₂)Unbalanced Equation: H2+O2→H2OH2+O2→H2O
Steps to Balance:
- Balance hydrogen (H). There are 2 hydrogens on the left and 2 on the right. H2+O2→2H2OH2+O2→2H2O
- Balance oxygen (O). There are 2 oxygens from O₂ and 2 × 1 = 2 oxygens from H₂O, totaling 2 oxygens on the right. The equation is already balanced for oxygen.Now, the equation is balanced with 2 hydrogens and 2 oxygens on both sides.
Formatting and Cleaning Up Combined Data
There are several tools and software options commonly used for balancing chemical equations, catering to different needs from basic balancing to more advanced chemical calculations. Here are some popular choices:
- Chemical Equation Balancer Websites:
- WebQC’s Equation Balancer: A simple online tool that balances chemical equations. It’s user-friendly and suitable for basic balancing tasks.
- ChemBalancer: Another web-based tool that balances equations and provides explanations for each step in the process.
2.Chemistry Software:
- ChemDraw: Widely used for chemical structure drawing, but also includes tools for balancing equations and performing stoichiometric calculations.
- ChemOffice: Offers a suite of tools including equation balancing, molecular modeling, and chemical property prediction.
- ACD/ChemSketch: Provides tools for drawing chemical structures and balancing equations, along with other chemical calculations.
3. Scientific Calculator Apps:
- Many scientific calculators have built-in functions for balancing equations, especially those designed for chemistry or scientific purposes.
3. Chemistry Apps:
- Many scientific calculators have built-in functions for balancing equations, especially those designed for chemistry or scientific purposes.
- Mobile apps like “Chemical Equation Balancer” for Android or iOS provide on-the-go equation balancing capabilities.
4. Chemistry Learning Platforms:
- Platforms like Khan Academy and ChemCollective offer interactive tools and tutorials for learning and practicing equation balancing.
Conclusion
FAQs
Q: 1What is balancing a chemical equation?
Ans::Balancing a chemical equation involves adjusting coefficients (numbers in front of compounds) to ensure the same number of atoms of each element on both sides of the equation. This process maintains the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Q: 2Why is balancing chemical equations important?
Ans: Balancing chemical equations is crucial because it ensures the accuracy of chemical reactions. By balancing equations, we confirm that the same amount of each type of atom exists before and after a reaction, providing a clear picture of reactants and products in stoichiometric proportions.
Q:3Can any chemical equation be balanced?
Ans: Yes, every chemical equation can be balanced if the correct approach is used. This involves systematic adjustment of coefficients to ensure conservation of mass and atoms. However, some equations may be more complex and require more steps or advanced techniques to balance accurately.
Q: 4Are there tools or software to help balance chemical equations?
Ans: Yes, there are various tools and software available to assist in balancing chemical equations, ranging from simple online equation balancers to sophisticated chemistry software packages. These tools automate the process and often provide step-by-step guidance, making equation balancing more efficient and accurate for students and professionals alike.






