Valency Chart
Valency chart, frequently alluded to as a valence electron chart or Lewis electron speck chart, could be a visual representation of the valence electrons of iotas in a chemical compound. It makes a difference in understanding how iotas bond with each other to make atoms by sharing, picking up, or losing electrons. Here’s a basic clarification of how a valency chart works:
- About Valency Chart:
- Understanding Valency Charts: A Visual Guide to Chemical Bonding
- Valency of First 30 Elements
- 4. Components of a Valency Chart:
- 5. Using a Valency Chart:
- 6.Unveiling the Magic: How to Use a Valency Chart:
- Unlocking the World of Chemistry: Applications and Insights:
- Valency and Oxidation State
- Key Points:
- Exploring Electron Interaction:
- Conclusion: A Dance of Electrons
- FAQ’s
About Valency Chart:
A valency chart, too known as a Lewis electron speck chart or Lewis structure, could be a visual representation utilized in chemistry to show the course of action of valence electrons within the furthest vitality level (valence shell) of molecules inside a molecule. Valency charts give bits of knowledge into how iotas bond together to create compounds by sharing, picking up, or losing electrons. They offer assistance us get it the dispersion of electrons and the coming about chemical behavior of components and atoms.
Understanding Valency Charts: A Visual Guide to Chemical Bonding
Chemical holding lies at the heart of understanding how components combine to make compounds with interesting properties. Valency charts, too known as Lewis electron dab graphs, give a visual representation of how molecules share, gain, or lose electrons to realize a steady electron setup. In this comprehensive direct, we dig into the points of interest of valency charts, their components, and how they offer assistance us unwind the puzzles of chemical holding.
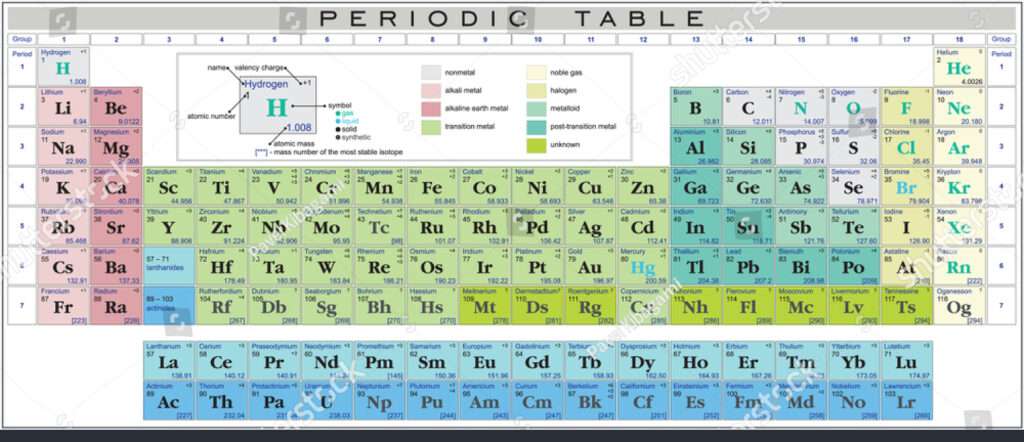
Valency of First 30 Elements
| Element | Atomic Number | Valency |
| Valency of Hydrogen | 1 | 1 |
| Valency of Helium | 2 | 0 |
| Valency of Lithium | 3 | 1 |
| Valency of Beryllium | 4 | 2 |
| Valency of Boron | 5 | 3 |
| Valency of Carbon | 6 | 4 |
| Valency of Nitrogen | 7 | 3 |
| Valency of Oxygen | 8 | 2 |
| Valency of Fluorine | 9 | 1 |
| Valency of Neon | 10 | 0 |
| Valency of Sodium (Na) | 11 | 1 |
| Valency of Magnesium (Mg) | 12 | 2 |
| Valency of Aluminium | 13 | 3 |
| Valency of Silicon | 14 | 4 |
| Valency of Phosphorus | 15 | 3 |
| Valency of Sulphur | 16 | 2 |
| Valency of Chlorine | 17 | 1 |
| Valency of Argon | 18 | 0 |
| Valency of Potassium (K) | 19 | 1 |
| Valency of Calcium | 20 | 2 |
| Valency of Scandium | 21 | 3 |
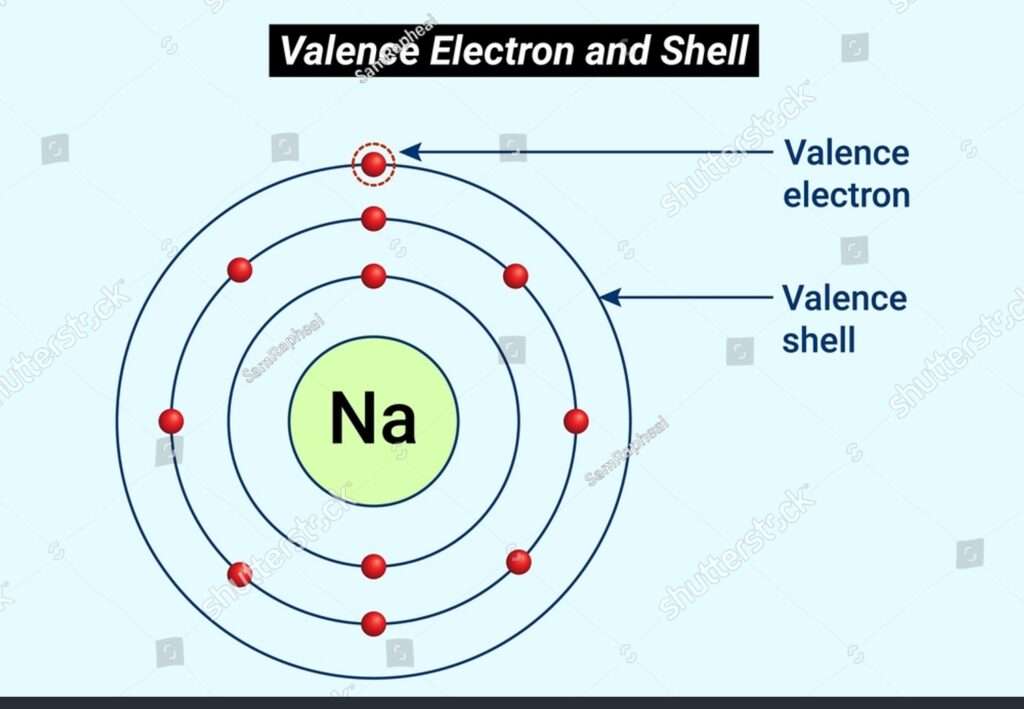
Components of a Valency Chart:
Component Images: The chemical images of the components included within the compound are put at the center of the chart. For case, H for hydrogen or O for oxygen.
Valence Electrons: Valence electrons are the furthest electrons in an atom’s electron setup. These electrons decide an atom’s reactivity and its propensity to make bonds with other particles.
Electron Dabs: The valence electrons are spoken to by specks put around the component image. Each side of the image can hold one electron dab, and they are frequently orchestrated in sets.
Using a Valency Chart:
Tally Valence Electrons: Decide the number of valence electrons for each particle by alluding to its position within the periodic table. Components within the same bunch (column) regularly have the same number of valence electrons.
Put Electron Specks: Begin placing the electron specks around the component image. You’ll be able put one speck on each side some time recently matching them. Once you have got two specks on a side, move to the another side.
Share or Exchange Electrons: The course of action of dabs makes a difference you visualize how particles can share, pick up, or lose electrons to realize a steady electron arrangement. Particles point to have a full peripheral shell, frequently with 8 electrons (octet run the show).
Frame Chemical Bonds: Components can share electrons (covalent bond) or exchange electrons (ionic bond) to fill their furthest shells. The sharing or exchange leads to the arrangement of chemical compounds.
Take after Rules: There are particular rules for making electron dab charts, such as Hund’s run the show and the octet run the show, which direct the situation of electrons to guarantee soundness.
Unveiling the Magic: How to Use a Valency Chart:
Tally Valence Electrons: Start by tallying the number of valence electrons for each molecule based on its position within the intermittent table. For case, hydrogen (H) has one valence electron, whereas oxygen (O) has six.
Put Electron Dabs: Speak to valence electrons as specks around the component image. Each side can hold a most extreme of two electrons, which are regularly set side by side (speaking to a combine).
Visualize Holding Conceivable outcomes: The course of action of electron dots permits you to imagine how molecules can accomplish steadiness by sharing, picking up, or losing electrons. The objective is to achieve a full peripheral electron shell, frequently comprising eight electrons (the octet run the show).
Predict Molecular Behavior: By analyzing the valency chart, you’ll be able expect how molecules associated to make bonds. Components can share electrons (covalent holding) or exchange electrons (ionic holding) to realize a steady electron setup.
Take after Valency Rules: Valency charts follow particular rules, such as Hund’s run the show and the octet run the show, which direct the situation of electrons. These rules guarantee that molecules accomplish the foremost steady electron course of action conceivable.
Unlocking the World of Chemistry: Applications and Insights:
Chemical Holding: Valency charts are the key to understanding how particles connect powers to make compounds with differing properties.
Atomic Geometry: The course of action of electron dabs offers experiences into the shape and geometry of particles.
Response Prediction: By analyzing valency charts, you’ll be able foresee the result of chemical responses and the arrangement of unused compounds.
Electron Exchange: In ionic bonding, valency charts outline how electrons are exchanged between particles to attain stability.
Sharing Electrons: In covalent holding, valency charts exhibit how iotas share electrons to attain a full external shell.
Valency and Oxidation State
Within the captivating domain of chemistry, the concepts of valency and oxidation state play significant parts in unraveling the behavior of iotas and atoms. These concepts give experiences into the ways electrons connected inside compounds, forming the properties and responses of matter. Let’s set out on a travel to get it the noteworthiness of valency and oxidation state within the complex move of electrons.
Defining Valency:
Valency, too known as valence, alludes to the combining capacity of an component, demonstrating the number of electrons an molecule can share, pick up, or lose to realize a steady electron setup.
Key Points:
Valency is decided by the number of valence electrons an molecule has, ordinarily found within the peripheral vitality level.
The respectable gasses have a valency of zero, as they as of now have a total set of valence electrons.
Components tend to make compounds to achieve the steady electron setup of respectable gasses (octet run the show).
Valency administers the sort and number of bonds an particle can frame in a compound.
Exploring Electron Interaction:
Shared Electrons: In covalent bonds, iotas share electrons to realize a steady electron arrangement. The valency of each particle decides the number of electrons it can contribute to the shared bond.
Electron Exchange: In ionic bonds, particles exchange electrons to attain a full external shell. The contrast in valency between two components decides the number of electrons exchanged.
Redox Responses: Oxidation state changes are central to redox (reduction-oxidation) responses. One component loses electrons (oxidation), whereas another picks up electrons (lessening).
Adjusting Conditions: Understanding valency and oxidation state makes a difference adjust chemical conditions by guaranteeing that the number of electrons picked up and misplaced in a response are rise to.
Conclusion: A Dance of Electrons
Valency and oxidation state give a more profound understanding of how electrons connected to form the complex world of chemistry. Valency directs an atom’s holding capacity, whereas oxidation state uncovers the genuine electron dissemination. Together, these concepts direct us through the complex ways of covalent and ionic bonding, redox responses, and the elemental standards that oversee the behavior of matter. Within the amazing ensemble of the nuclear move, valency and oxidation state serve as our guideposts, making a difference us interpret the dialect of electrons and the enchantment they weave.
FAQ'S
Valency, also known as valence, is a term used in chemistry to describe the combining capacity of an element or atom with other elements. It indicates the number of electrons an atom can gain, lose, or share in order to achieve a stable electron configuration similar to that of a noble gas.
Carbon includes a valency of four. This implies that a carbon molecule can frame up to four covalent bonds with other iotas. Valency is decided by the number of electrons within the furthest vitality level of an particle, which is additionally known as the valence shell. Within the case of carbon, it has four electrons in its furthest shell.
Carbon’s valency of four makes it exceedingly flexible and shapes the premise for the complexity of natural chemistry. It can form single, twofold, or indeed triple bonds with other carbon particles or with iotas of other components. This capacity to create different bonds is what permits carbon to form different and perplexing atomic structures, which are fundamental for the tremendous assortment of natural compounds found in nature and utilized in different mechanical forms.
Tetravalency alludes to the property of an iota, especially carbon, to have a valency of four. This implies that the iota can frame up to four covalent bonds with other iotas in arrange to attain a stable electron arrangement. Tetravalency may be a critical concept in chemistry and is especially related with carbon due to its capacity to make four covalent bonds.
Within the setting of carbon:
Four Valence Electrons: Carbon has four electrons in its peripheral vitality level (valence shell).
Arrangement of Covalent Bonds: Carbon can share its four valence electrons with other iotas, permitting it to make up to four covalent bonds. These bonds can be single, twofold, or indeed triple bonds, depending on the sort of atom being shaped.
Tetravalency alludes to the property of an iota, especially carbon, to have a valency of four. This implies that the molecule can shape up to four covalent bonds with other particles in arrange to attain a stable electron arrangement. Tetravalency may be a critical concept in chemistry and is especially related with carbon due to its capacity to make four covalent bonds.
Within the setting of carbon:
Four Valence Electrons: Carbon has four electrons in its furthest vitality level (valence shell).
Arrangement of Covalent Bonds: Carbon can share its four valence electrons with other molecules, permitting it to make up to four covalent bonds. These bonds can be single, twofold, or indeed triple bonds, depending on the sort of particle being shaped.






