The Gattermann-Koch reaction, a notable naming reaction, finds industrial application in the synthesis of benzaldehyde. The collaborative efforts of Ludwig Gattermann and Julius Arnold Koch led to its development. This reaction is primarily employed to introduce a formyl group (-CHO) onto aromatic systems. It’s worth noting that the Gattermann-Koch reaction is not effective when applied to substrates of phenol and phenol ether. When zinc chloride serves as the catalyst in this reaction, copper(I) chloride assumes the role of a crucial co-catalyst. This article delves into comprehending the Gattermann-Koch reaction’s mechanism, recognizing its limitations, and provides relevant example queries.
What is the Gattermann – Koch reaction?
This is a chemical transformation used in organic synthesis to introduce a formyl group (-CHO) onto aromatic compounds. This reaction enables the conversion of various aromatic compounds into their corresponding benzaldehyde derivatives. It was named after its developers, Ludwig Gattermann and Julius Arnold Koch.
The reaction involves the use of a catalyst, often zinc chloride (ZnCl2), and a co-catalyst, usually copper(I) chloride (CuCl), which is essential for the reaction to proceed effectively. The reaction typically takes place under specific conditions, often involving the use of hydrogen cyanide (HCN) as a source of the formyl group, along with a Lewis acid, such as hydrogen chloride (HCl), to facilitate the reaction.
This is a significant chemical process that allows for the direct introduction of a formyl group onto aromatic compounds, resulting in the synthesis of benzaldehyde derivatives.
An example of Gattermann – Koch is as given below:
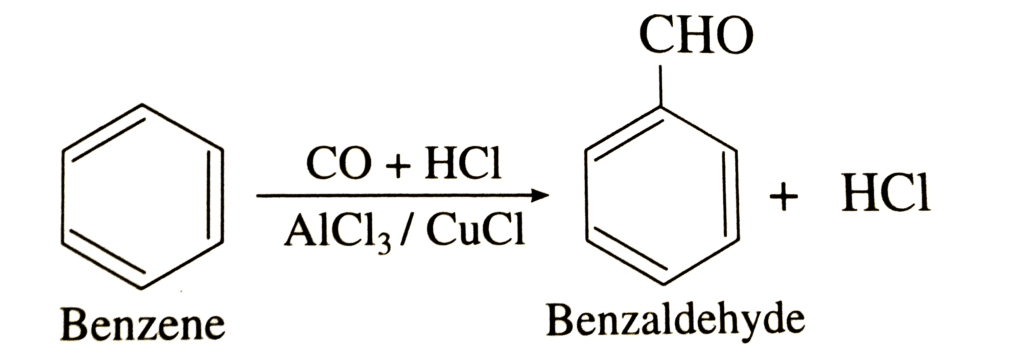
Mechanism of Gattermann – Koch Reaction
The Gattermann–Koch reaction mechanism may be broken down into three steps:
Step 1: Forming Formyl Chloride
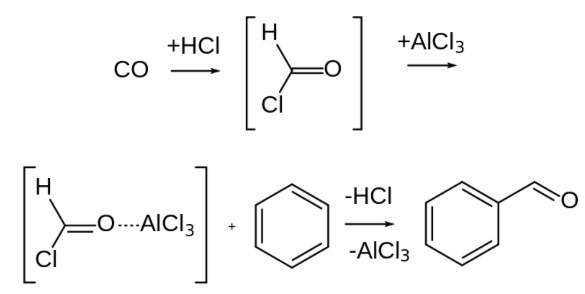
The initial stage of this reaction involves generating reactive entities that can subsequently engage with the aromatic ring. Carbon monoxide can accept a proton from hydrochloric acid due to its nature as a Lewis base. This leads to the creation of a positively charged molecule with several resonance forms. Among these resonance structures, one involves a positive charge on carbon, accounting for the reactivity of the composite. When it interacts with the aromatic ring, this entity can act as an electrophile.
However, a more plausible scenario is that the chloride ion within hydrochloric acid acts as a nucleophile, initiating a reaction with this species.
Step 2:
Upon the addition of a Lewis acid such as aluminum chloride to the species, a rapid expulsion of a chloride ion takes place. Consequently, the species reverts back to the formyl cation state, regaining its reactive nature.
Step 3:
At the aromatic ring, an electrophilic aromatic substitution occurs. The formyl cation receives an electron pair from the aromatic ring, which serves as a nucleophile. The loss of aromaticity is quickly restored when a proton is ejected.
As a result of the Gattermann – Koch reaction, the formyl group is linked to the aromatic ring. Benzaldehyde is produced when benzene is treated with carbon monoxide and hydrochloric acid in the presence of aluminium chloride, as illustrated in the above process.
Limitations of Gattermann – Koch reaction
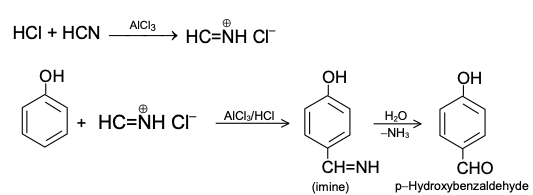
The scope of this reaction is limited to alkylbenzenes due to the absence of suitable substrates. To address this limitation, Gattermann developed a modified approach enabling the formylation of phenols, phenolic ethers, and heteroaromatic compounds like pyrroles and indoles.
However, a notable drawback of the Gattermann method was its reliance on anhydrous hydrogen cyanide (HCN). To circumvent this challenge, R. Adams introduced a technique in which HCN is generated in situ from zinc cyanide and hydrochloric acid. This innovation, known as the Adams modification, has gained widespread use in organic synthesis as a practical alternative.
Applications of Gattermann - Koch reaction
The Gattermann reaction, which involves the formylation of aromatic compounds, has several practical applications in the field of organic synthesis. Some of the notable applications include:
Benzaldehyde Synthesis: Undoubtedly, one of the paramount applications of the Gattermann- reaction lies in the industrial production of benzaldehyde. This compound, known for its versatile nature, serves as a crucial flavoring agent and fragrance in various industries. Moreover, it assumes the role of a pivotal intermediate in the synthesis of a diverse array of chemicals, encompassing pharmaceuticals and agrochemicals alike.
Pharmaceutical Industry: The Gattermann- reaction assumes a pivotal role within the pharmaceutical sector. Chemists strategically incorporate it in synthesizing intermediates for pharmaceuticals compounds, thereby seamlessly bridging fundamental chemistry with the realm of drug development. The remarkable capacity to introduce formyl groups onto aromatic rings takes on paramount significance.
Perfume and Fragrance Industry: Aromatic compounds play a crucial role in shaping perfumes and fragrances, standing as an integral element in their formulation. Chemists play an active role by utilizing the process of formylating aromatic rings to purposefully modify the scent profile of compounds. This deliberate alteration propels the creation of novel and distinctive fragrances that truly captivate the senses.
Agrochemicals: Utilizing formylation on aromatic compounds finds application in synthesizing agrochemicals, including pesticides and herbicides. These compounds frequently demand precise functional groups for their effectiveness, which chemists can introduce through the Gattermann-Koch reaction. This process empowers chemists to actively incorporate functional groups critical to their activity.
Material Science: Transitioning to the realm of material science, aromatic compounds bearing formyl groups assume a pivotal role. They serve as invaluable precursors for synthesizing a diverse array of materials, prominently encompassing polymers and resins.
Natural Product Synthesis: Chemists frequently utilize the Gattermann- reaction to synthesize natural products containing aromatic rings featuring formyl groups. This application proves especially invaluable in the realm of total synthesis, where chemists strive to replicate intricate natural compounds within the laboratory setting.
FAQs
The Gattermann- reaction was developed by chemists Ludwig Gattermann and Julius Arnold Koch
The introduction of a formyl group onto aromatic compounds is valuable because it imparts new chemical functionalities and opens up opportunities for the creation of compounds with distinct properties and applications.
The reaction typically employs zinc chloride (ZnCl2) as the catalyst and copper(I) chloride (CuCl) as a co-catalyst. These compounds facilitate the formylation process.
Originally, the reaction was limited to alkylbenzenes, but modifications have expanded its applicability to include phenols, phenolic ethers, and heteroaromatic compounds like pyrroles and indoles.
The Adams modification is an enhancement of the Gattermann-reaction. It involves generating hydrogen cyanide (HCN) in situ from zinc cyanide and hydrochloric acid, making the process safer and more practical compared to using hazardous anhydrous HCN.
The Gattermann-reaction finds applications in industries such as perfumery, pharmaceuticals, agrochemicals, and materials science. It is used to synthesize compounds for fragrances, pharmaceutical intermediates, agrochemicals, and more.
The reaction is employed in the synthesis of natural products containing aromatic rings with formyl groups. This is particularly useful in total synthesis, where chemists aim to recreate complex natural compounds in the laboratory.
Conclusion
This reaction demonstrates the ingenuity of chemists in devising inventive methodologies for organic synthesis. It has evolved from its inception of formylating alkylbenzenes to encompass a wider range of aromatic substrates, thus imprinting an enduring influence on the field. The subsequent Adams modification not only heightened its practicality and safety but also reflected the dynamic evolution of chemical techniques. As the realm of chemistry continues its advancement, the reaction persists as a foundational instrument within the repertoire of synthetic chemists. It empowers the meticulous crafting of intricate molecules with exceptional precision.






