The contrast between soap and detergent lies in their composition. Soap is formed from metal salts of fatty acids, whereas detergent is created using potassium or sodium salts of extended alkyl chains. Soaps are derived from natural sources such as plant oils or animal fat-derived acids, while detergents are synthetic and artificially produced. However, detergents have lower biodegradability. Detergents are preferred over soaps for washing clothes in hard water due to their superior cleansing properties.
Difference Between Soaps & Detergents
Chemical Composition and Structure:
Soap:
Soap is ordinarily made from common fixings like plant oils (such as coconut, palm, olive) or creature fats. The method of saponification includes responding these oils or fats with an soluble base (more often than not sodium hydroxide or potassium hydroxide) to create cleanser particles. The structure of soap particles comprises of a polar “head” (hydrophilic) and a nonpolar “tail” (hydrophobic).
Detergent:
Detergent, on the other hand, are manufactured compounds inferred from petrochemicals. They are made through complex chemical forms. Detergent atoms too have a polar head and a nonpolar tail, comparative to soap, but the particular chemical structure can change broadly based on the sort of detergent.
Biodegradability:
Soap:
Soap is typically more environmentally friendly than many synthetic detergents because its molecules can be naturally broken down by microorganisms in the environment, reducing its impact. Nevertheless, the biodegradability of soap might be influenced by the existence of particular additives or impurities.
Detergent:
Although certain contemporary detergents are formulated to be biodegradable, numerous conventional detergents consist of components that resist decomposition by natural mechanisms. These non-biodegradable detergents have the potential to worsen pollution and negatively impact aquatic ecosystems.
Performance in Hard Water:
Soap:
Soap tends to create insoluble salts (known as precipitates) when used in hard water, which is rich in calcium and magnesium ions. These precipitates can lead to the formation of a residue on surfaces and clothing, commonly referred to as “soap scum.”
Detergent:
Detergents, which are synthetic in nature, are engineered to function efficiently in both soft and hard water. They do not undergo precipitation with the calcium and magnesium ions found in hard water. Consequently, detergents are the preferred choice for laundering clothes and cleaning surfaces in regions characterized by hard water conditions.
Cleaning Efficiency:
Soap:
Soap is compelling at expelling earth, oils, and oil from surfaces due to its hydrophobic tails that can connected with nonpolar substances. Be that as it may, soap’s adequacy can be prevented in difficult water due to the arrangement of soap scum.
Detergent:
Detergents are formulated to maintain their cleaning efficiency even in the presence of minerals found in hard water. Their synthetic nature allows for precise control over their properties, making them versatile cleaning agents.
Applications:
Soap:
Soaps are commonly used for personal hygiene, such as washing hands and bathing. They are also used in household cleaning products.
Detergent:
Detergents have a wide range of applications, including laundry detergents, dishwashing detergents, household cleaners, and industrial cleaning solutions.
Preparation of Soap
Saponification is a commonly used technique in soap production, which converts oils and fats into soap, glycerin, and water. By heating oils and fats and then mixing them with an alkali (like sodium hydroxide or potassium hydroxide), a chemical reaction takes place, giving rise to the creation of soap molecules. This chemical process is a form of hydrolysis, where the alkali breaks the ester bonds found in triglyceride molecules (the primary components of oils and fats). As a result of this reaction, the fatty acids and glycerol within the triglycerides separate.
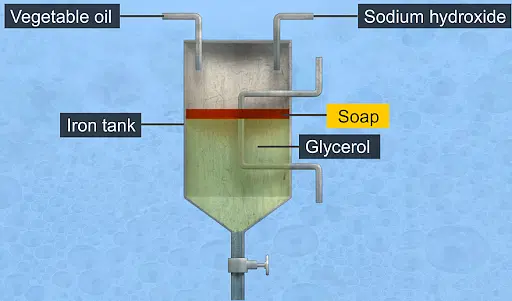
The reaction equation for saponification can be represented as follows:
Triglyceride + Alkali → Soap + Glycerol
How do Soaps & Detergents Help in Cleaning Out Dirt?
The process of cleaning a soiled surface comprises four primary stages. Initially, the surface is moistened and saturated with water. Subsequently, soap or detergent is administered onto the stained region to facilitate absorption. Soaps and detergents, also recognized as surfactants or surface-active agents, encompass molecules that possess surface-active properties. These molecules inherent in soaps and detergents dissolve effectively in water.
Things To Keep In Mind
- Soaps consist of sodium salts derived from fatty acids or carboxylic acids possessing lengthy chains. Detergents, on the other hand, are constituted of sodium salts originating from benzene sulfonic acids that exhibit extended chains.
- While soaps exhibit biodegradability, certain types of detergents lack this property. Detergents exhibit a more potent cleaning efficacy when compared to soaps.
- Both soaps and detergents function as surfactants, making them valuable for the removal of dirt and impurities.
- The manufacturing process of soaps involves the utilization of fats, oils, or their corresponding fatty acids, neutralized through the use of robust alkali compounds.
- The chemical transformation responsible for the creation of soap is known as saponification.
Sample Questions
- Soaps are made from fatty acids, while detergents are made from sulfonated hydrocarbons.
- Soaps are biodegradable, while some detergents are not.
- Soaps can form scum in hard water, while detergents do not.
- Soaps are less effective in hard water and saline water, while detergents are more effective.
Soaps are typically prepared through saponification, where fats and oils are reacted with an alkali. Detergents are synthesized using various chemical processes involving sulfonation and other reactions.
A common soap example is sodium stearate, while sodium dodecylbenzenesulfonate (SDBS) is an example of a detergent.
Soaps are generally biodegradable, whereas some detergents, particularly those containing branched or alkylbenzene chains, can be non-biodegradable and environmentally persistent.
Detergents often possess a stronger cleaning effect than soaps due to their ability to function effectively in hard water and under different pH conditions.
Conclusion
In conclusion, understanding the distinctions between soaps and detergents is crucial for comprehending their applications in cleaning and their impact on the environment. Soaps, derived from natural sources through saponification, are sodium salts of fatty acids. They exhibit biodegradability and are effective in certain cleaning scenarios. On the other hand, detergents, often synthetically produced from petroleum-based materials, are sodium salts of benzene sulfonic acids. While detergents can offer superior cleaning efficacy, their environmental impact varies based on biodegradability.






