Calcium Carbonate, denoted chemically as CaCO3, is a ubiquitous and versatile inorganic compound with a plethora of applications across various industries and scientific disciplines. Renowned for its remarkable stability and lack of odor, Calcium Carbonate stands as one of the most recognizable and non-toxic chemicals.
Present in a variety of natural states, Calcium Carbonate stands as a significant component within minerals like limestone, marble, and chalk. These geological configurations have served as crucial reservoirs of this compound over extended periods, offering a vital resource for a wide array of utilitarian applications. Its distribution extends beyond the terrestrial crust, encompassing marine organisms’ shells and the exoskeletons of various creatures. This presence underscores its fundamental significance within ecological frameworks.
What is CaCO3?
CaCO3 is the chemical formula for calcium carbonate, which is an inorganic chemical compound. It is composed of one calcium (Ca) atom, one carbon (C) atom, and three oxygen (O) atoms. Calcium carbonate is a common substance found in various forms in nature, such as limestone, marble, and chalk. It has a wide range of applications in industries like construction, agriculture, pharmaceuticals, and more, due to its stability, versatility, and non-toxic nature.
In its various crystalline forms, Calcium Carbonate manifests in diverse appearances, ranging from the lustrous elegance of marble to the unassuming texture of limestone and the dusty softness of chalk. This adaptability in structure is deeply intertwined with its widespread presence in both geological formations and biological systems.
Geologically, Calcium Carbonate contributes to the formation of extensive deposits, making its home within minerals such as limestone and marble, sculpting landscapes and shaping the very foundations of our Earth’s crust. Its affinity for precipitating from aqueous solutions under specific conditions has led to the creation of awe-inspiring stalactites and stalagmites in caves, a testament to its role in Earth’s intricate geological processes.
- Calcium carbonate is classified as a calcium salt derived from carbonic acid. Its presence is evident in various forms such as chalk, limestone, and marble. Despite their distinct physical characteristics, these manifestations share identical chemical compositions.
- This compound holds practical value in food preservation and pharmaceutical production. It finds application in the preservation of edibles and contributes to the creation of medicinal formulations.
- However, it’s crucial to exercise caution as excessive intake of calcium carbonate can pose health risks. In addition, its consumption has the potential to lead to digestive issues and should be approached judiciously.
Calcium Carbonate Formula
Calcium carbonate is a chemical substance with the formula CaCO3.
- Instances of this compound’s natural presence encompass materials like marble, chalk, limestone, shells, calcite, and pearls, among others.
- It assumes the form of a white, immiscible powdery substance.
- Within the medical realm, it serves roles as an antacid and a calcium additive.
- Moreover, it finds practical utility as a cosmetic filler.
- Across the industrial sector, it plays a substantial role, functioning as a constituent in quicklime, cement, and construction materials like marble.
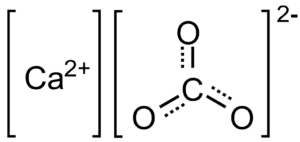
Calcium Carbonate Structure
The structure of Calcium Carbonate is as follows:
Forms of Calcium Carbonate
Calcite, aragonite, and vaterite are the three polymorphic forms of calcium carbonate.
Calcite, known for its exceptional stability and low solubility, is a mineral that plays a pivotal role in rock formation, prevalent in sedimentary, metamorphic, and igneous rocks. Its presence is prominent in sedimentary rock formations like limestone and marble. Its multifaceted utility extends to domains like construction materials, soil enhancement in agriculture, pharmaceuticals, and a diverse range of applications.
Aragonite, while less stable and more soluble than calcite, emerges as a result of both biological and physical processes. It forms through precipitation in marine and freshwater settings. Aragonite serves as a source of nutrients for marine life and contributes to maintaining pH levels within their natural parameters. Beyond this ecological role, it proves invaluable in removing pollutants such as zinc, cobalt, and lead from polluted wastewater.
Vaterite, distinguished by its hexagonal morphology, occupies a position intermediate to calcite and aragonite in terms of stability and solubility. Its origins encompass springs, organic tissues, urinary calculi, gallstones, and botanical sources. With applications spanning regenerative medicine and a spectrum of personal care products, vaterite finds relevance in diverse domains.
Preparation of Calcium Carbonate (CaCO3)
The extraction of CaCO3 occurs through mining and quarrying methods. Another approach involves its preparation by utilizing calcium hydroxide (also known as slaked lime, Ca(OH)2) and carbon dioxide as primary raw materials.
Passing carbon dioxide through slaked lime leads to the formation of calcite.
Ca(OH)2 + CO2 →→ CaCO3 + H2O
Calcite can also be acquired through the addition of calcium chloride to sodium carbonate.
CaCl2 + Na2CO3→CaCO3 + 2NaCl
Calcium carbonate (CaCO3) can be synthetically produced by combining calcium oxide (quicklime, CaO) with water to yield calcium hydroxide (Ca(OH)2). Subsequently, introducing carbon dioxide into this solution facilitates the formation of calcium carbonate.
CaO + H2O →→ Ca(OH)2
Ca(OH)2 + CO2 →→ CaCO3↓↓ + H2O
Properties of Calcium Carbonate (CaCO3)
The properties of Calcium Carbonate are as follows:
- It presents itself with a white, powdery consistency.
- The molecular weight or molar mass of Calcium carbonate (measured in g/mol) equates to 100.0869 g/mol.
- Calcium carbonate has a density of 2.71 g/cm³.
- At a boiling point of 899°C/1200 K, it decomposes, and its melting point stands at 825°C.
- When subjected to dilute acids, Calcium carbonate reacts to produce carbon dioxide as a resultant by-product.
Uses of Calcium Carbonate (CaCO3)
The uses of Calcium Carbonate are listed below:
- Calcium carbonate finds extensive applications within the paper, paints, and pulp sectors.
- In the realm of cosmetics, it serves as both a pigment and filter, delivering a whiter and superior pigment quality compared to alternative minerals.
- Its utility extends to tablet production and as an antacid for alleviating stomach discomfort and heartburn.
- Furthermore, it assumes a crucial role in the manufacture of construction materials, whether as a component in marble or as an integral ingredient in cement.
- In instances of calcium deficiency, it functions as a calcium supplement.
The sugar refining process benefits from its involvement.
In agriculture, it acts to neutralize acidic soil.
Water and sewer plant treatment procedures utilize it to counteract acidity and eliminate impurities.
Commercial Production of Calcium Carbonate
Commercially, there are two variants of Calcium Carbonate available. These two forms primarily vie for supremacy within the industrial market based on factors such as particle size and the characteristics attributed to a particular product.
- Ground calcium carbonate is sourced and processed from naturally existing deposits.
- The crystalline structure of GCC is characterized by irregular rhombohedral shapes and displays a broader range of sizes.
- Precipitated calcium carbonate is generated through chemical precipitation employing a carbocation method or as a secondary outcome of certain large-scale chemical procedures.
- The configuration of PCC crystals varies based on the specific product, showcasing a more uniform and consistent particle structure, along with a confined range of sizes.
- PCC showcases finer particles that possess greater purity, exhibit reduced abrasiveness, and showcase heightened brightness when compared to GCC.
FAQs
Limestone (calcium carbonate CaCO 3) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of CaCO 3. Limestone forms when these minerals precipitate out of water containing dissolved calcium.
It plays an important role in construction, be it as a building material (marble) or as an ingredient in cement. It is used in medicinal industries which manufacture antacids, tablets which are made of base materials etc. It is used as calcium supplement. It is used in the manufacture of paints, paper, plastics, etc.
Conclusion
In conclusion, Calcium Carbonate stands as a multifaceted compound with an extensive array of applications and significance across various industries and scientific disciplines. Its role in geological formations, ecological systems, industrial processes, and even daily life underscores its pervasive presence and impact. From its extraction through mining and quarrying to its synthetic production through chemical reactions, Calcium Carbonate’s versatility shines through.






